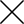Scientists on Monday gave the first-ever injection of an experimental coronavirus vaccine to volunteers in the US.
Doses of mRNA-1273 into the arms of healthy participants at Kaiser Permanente Washington Research Institute (KPWHRI) this week began the first-stage study of a potential COVID-19 immunization.
The initial trial involves 45 people aged 18 to 55, including tech company operations manager Jennifer Haller—the study's first participant.
"We all feel so helpless.
This is an amazing opportunity for me to do something," the 43-year-old Seattelite told the Associated Press before getting jabbed.
She reportedly left the exam room with a smile, "feeling great."
The investigational vaccine, made by biotech company Moderna, does not contain any part of the actual coronavirus and cannot cause infection.
It instead includes a short segment of lab-grown messenger RNA.
Researchers are currently testing the safety of various doses to learn whether they produce an immune response.
They will keep an eye on any side effects and draw blood samples to look for clues.
"We don't know whether this vaccine will induce an immune response or whether it will be safe.
That's why we're doing a trial," Kaiser Permanente study leader Lisa Jackson said in a statement to the AP.
Efficiency in preventing COVID-19 will be determined at a later phase.
Recommended by Our Editors
Even if all goes well, a vaccine will not be available for widespread use for another 12 to 18 months, according to Anthony Fauci of the U.S.
National Institute of Health.
Still, "going from not even knowing that this virus was out there … to have any vaccine" in testing in about two months is unprecedented, Jackson said.
KPWHRI's vaccine research team previously conducted similar trials in the fight against "swine" (H1N1) and "bird" (influenza A) flu.