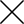The U.S.
Food and Drug Administration (FDA) this week approved Fitbit's emergency ventilator for use during the COVID-19 pandemic.
An automatic resuscitator, Fitbit Flow was developed in collaboration with Oregon Health & Science University emergency medicine clinicians and the Mass General Brigham Center for COVID Innovation.
"COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the health care systems caring for them," Fitbit CEO James Park said in a statement.
"We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for ventilators and help make a difference in the global fight against this virus."
A step up from standard hand-pumped resuscitator bags, Fitbit Flow uses technology to automate compressions and monitor patients.
The "intuitive and simple to use" device, the manufacturer said, aims to reduce the strain on hospital staff.
"Fitbit Flow is a great example of the incredible innovation that emerges when academia and industry employ problem-based innovation to respond quickly to an important need," according to David Sheridan, assistant professor of pediatric emergency medicine and co-director of emergency clinical innovation at Oregon Health & Science University.
"COVID-19 is a new illness and we still have much to learn about the progression, treatment, and potential recurrence of this disease," he continued.
"It's critical that we develop solutions that can help ensure our health systems have the equipment they need now, and in the future if we do see a resurgence of COVID-19."
Recommended by Our Editors
The same infrastructure that produces millions of wearables a year is being transformed into an assembly line for emergency devices.
Fitbit's goal is to supply these machines to global healthcare systems without traditional ventilators.